The novel technique for characterizing porous and microporous material in real environment.
APPLICATIONS OF THE METHOD OF STANDARD POROSIMETRY
Measurement of porosity, pore volume, pore radii and their distribution is important in many applications, products and processes. The Method of Standard Porosimetry (MSP) that was invited during research and development of electrochemical generator on the base of hydrogen-oxygen fuel cells within the framework of the Russian space program Buran-Energia.
The method is relatively simple and nondestructive it enables measurements in the widest range of pore sizes. MSP can also be used for evaluation of the contact angle and the hydrophilic-hydrophobic properties of multi-component materials.
In MSP any wetting liquid can be used as test liquid. The method allows operations in wide pressure and temperature ranges. Thus, MSP enables studying of very different porous materials and specimens in their real environment.
MSP does not require high pressures and is environmentally friendly. This method has been successfully utilized in all areas of materials investigation including the electrochemical industry.
MAIN FIELD OF APPLICATION:
- Batteries, fuel cells, electrolyzers and sensors,
- Electrodes, catalysts, active components,
- Membranes, separators, filters,
- Absorbents, ceramics, metalloceramics,
- Textiles, artificial and natural leather,
- Pharmaceuticals,
- Soils, wood, construction materials,
- Abrasives (e.g. grinding wheels or bars),
- Geological strata, etc.
CAPABILITIES OF STANDARD CONTACT POROSIMETRY
- Integral and differential distribution of pore volume as a function of the pore radius within range from ~1 nm to 3x105 nm;
- Average pore radius;
- Specific pore volume (porosity);
- Specific surface area (in the range from 10-3 to 103 m2/cm3);
- Distribution of pore surface as a function of the pore radius;
- Liquid distribution as a function of the values of the free binding energy between sample and liquid(in the range from 10-3 to 104 J/mole);
- Liquid distribution as a function of the values of the capillary pressure;
- Adsorption isotherms;
- Contact angle and its dependence on pore radius.
- Characteristics of porous structure depending on values of compression (up to 15 bar) and temperature (up to 80 оC)
- Can be applied to any material (including metals which easily form amalgams)
- Can be applied to compressible materials and powders without structure deformation
- Enables structural examination of swelling and compressible materials in their operational environment (water or other liquids, variouspressures, etc.)
- Provides comprehensive and easily interpret information, especially for swelling materials, multicomponent hydrophilic-hydrophobic systems, etc.
- Enables study of structural changes of specimens during different chemical and technological processes
- It does not employ highly toxic substances such as mercury, and does not cause errors due to variations of contact angle.
PRINCIPLES OF THE METHOD
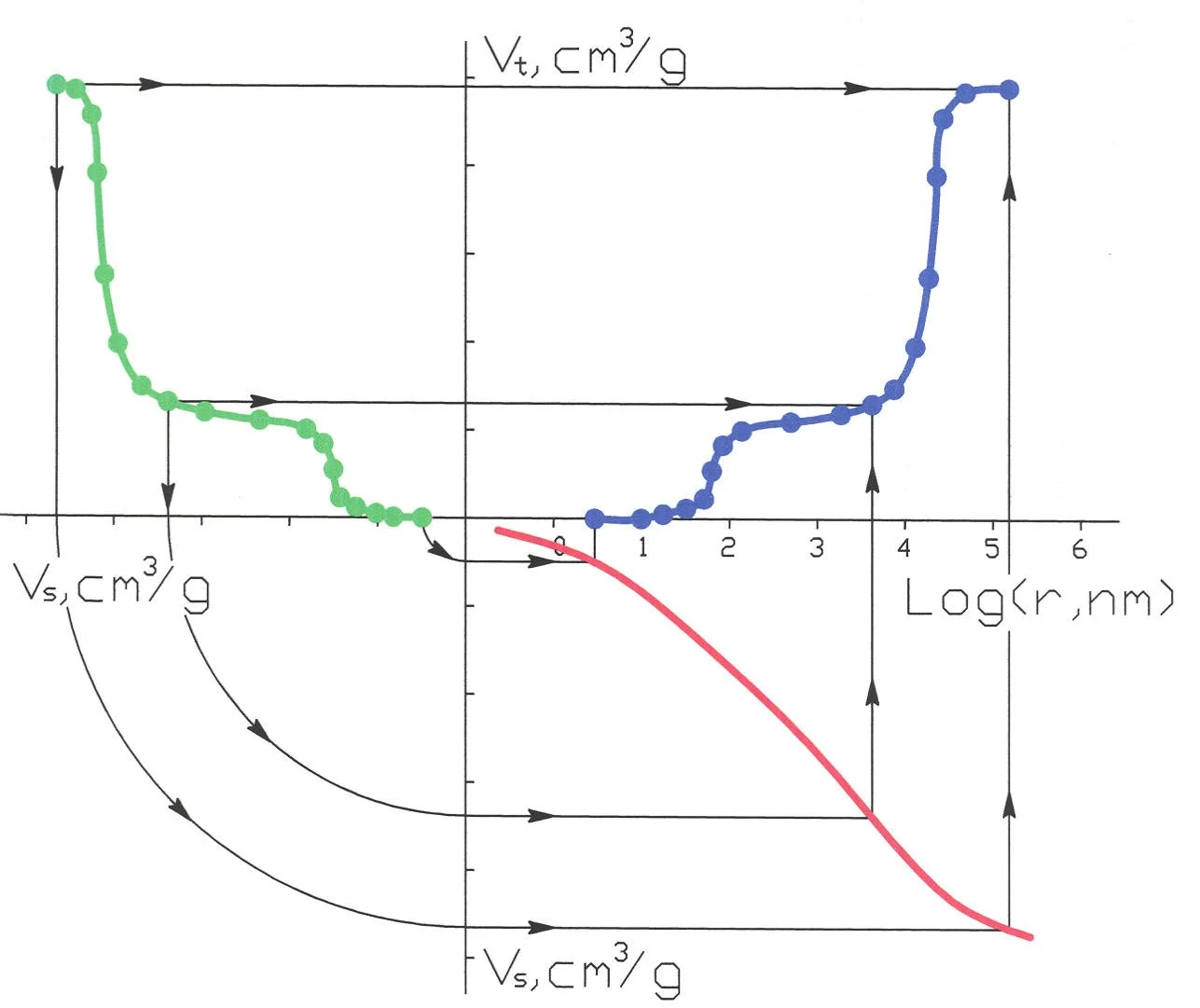
Picture 1
The method is based on the laws of the capillary equilibrium. If the porosimetric curve or porogram for one body (standard) is known, one can now obtain the porogram for a sample, by comparing volumes of liquid in the sample and in the standard at the condition of capillary equilibrium achieved by a firm contact of two porous bodies. The MSP technique measures the equilibrium dependence of the volume of the wetting liquid in sample (Vt) as a function of the liquid volume in standard (Vs). The amount of liquid in the samples is determined by weighing.
It is easier to understand how the method works with the help of the graphs on Picture 1. The green curve represents the dependence of the volume of liquid in sample (Vt) as a function of the liquid volume in standard (Vs) at the condition of capillary equilibrium. The red curve is an integral liquid distribution for the standard sample. The blue curve is an integral distribution of pore volume vs. pore radius which we are interested in. Follow to the arrows to understand the idea of the method
To start the process the sample and standards are dried, weighed and filled with liquid under vacuum treatment. The sample being tested is assembled between two standards in a stack and is brought into a firm contact with each other in the clamping device
- During the first stage a small portion of liquid is evaporated from this assembly.
- During the second stage the stack is disassembled and the samples are weighed individually.
- During the third stage the stack is reassembled and all operations are repeated several times until the liquid in the samples is completely evaporated.
It can be done using Our Automated Standard Porosimeter.
STANDARDS AND RANGE OF MEASUREMENT
Standards are complicated artificial porous bodies made in the shape of a disk.
The standards should possess three properties: to have pores in the maximum wide range of the sizes; not to change porous structure during storage and operation; to be firm enough and not to form amalgams with mercury.
Three methods are used to calibrate the standards: Capillary Condensation, Mercury Porosimetry (MMP) and Microscopy.
From the moment of the invention of MSP standards were continuously improved. At the beginning even two standards were used: one with macro and mesa pores, other with mesa and micro pores. Owing to creation of the modern standard the MSP has the widest range of measurement.
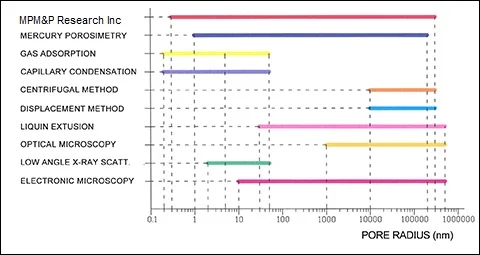
PICTURE 2
From comparison of ranges of measurements of various porosimetric methods (shown on Picture 2) it is not difficult to notice, that the most universal is the method of Standard Porosimetry (MSP) my MPM&P Research Inc. But it is not only advantage of the method. We'll try to show these advantages using comparisons of results of measurement of identical materials by various methods.
RESULTS OBTAINED BY MSP AND COMPARISON WITH OTHER METHODS
A comparison of the pore size distribution obtained by MSP and by other methods for ceramic Al2O3 based sample.
PICTURE 3
PICTURE 4
The data of Standard Porosimetry (MSP) are in a good agreement with the modern methods of research such as Atomic Force Microscopy (AFM), scanning electron microscopy (SEM). Distinctions with Mercury Porosimetry (MMP) should be related to a mistake in the setting of a contact angle of mercury. Usually it is considered that the contact angle of mercury with all materials is equal 140 degrees. However this is far from reality. For example, the angle can have value from 112 up to 150 for the same materials. Moreover it depends on the radius of a capillary.
At the same time, if a sample is strong enough, have no amalgamated inclusions and the contact angle for mercury is exactly known the results of the measurements with the MSP and MMP are practically identical.
Picture 4 shows the porograms measured by Method of Standard Porosimetry (MSP) and the Method of Mercury Porosimetry (MMP) for sufficiently firm and not amalgamated Ni and Ti electrodes. It can be seen there is a good agreement between the results, obtained by these two methods.
PICTURE 5
PICTURE 6
Sometimes MMP is used in examining samples with a low mechanical strength. To reveal the influence of high mercury pressures the results of measurements by MMP and MSP for such samples were compared at the Picture 5. As an example differential porograms are shown for a fibrous battery separator. It can be seen, that changes of the MMP curves take place according to deformation by high pressure of mercury.
Most metals can form amalgams with mercury. In the case when porous material contains these metals. Mercury porosimetry always gives increased results. You can see on the Picture 6 differential pore size distribution for a lead electrode of a lead oxide battery obtained by two methods. Here you can also see the negative influence of high pressure of mercury on the results. The lead oxide battery’s electrode is not strong enough and was destroyed by the pressure.
PICTURE 7
PICTURE 8
The knowledge of porous structure of components of Proton Exchange Membrane (PEM) Fuel Cells is important for understanding of mass and heat transfer process inside cells to increase specific energy or power of electrochemical devices in whole. The membrane
electrode assembly (MEA) is the core component of a fuel cell. It consists of the membrane, anode and cathode electrodes. Gas diffusion layers (GDL) are placed from both sides of MEA.
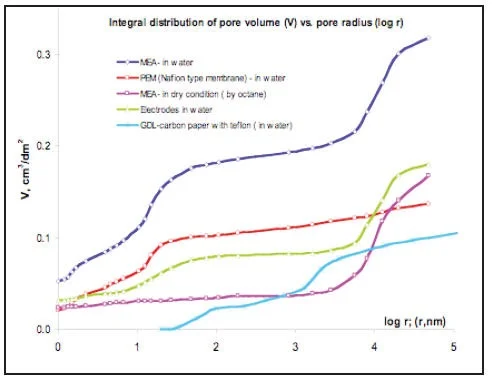
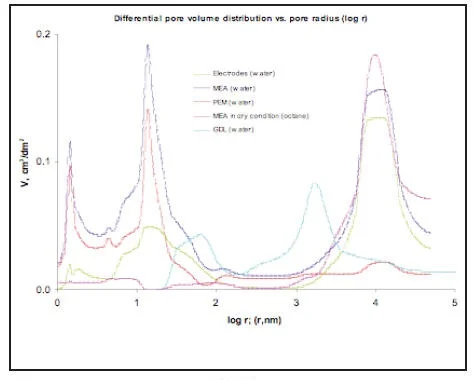
So the fuel cell represents a “sandwich” from five or more porous layers with different functions, porous structure and hydrophilic-hydrophobic properties. Complicated mass-transfer processes of hydrogen, oxygen and water with electrochemical reactions take place inside this “sandwich”. It is obvious, that the fuel cell overall performance depends on porous structure of each layer. MSP allows studying multi-layer systems in real environment: water, temperature, compression (Pictures 7,8,9).
Proton Exchange Membrane, which has the widest application in electrochemical devices doesn’t have any pores in a dry state. Porosity appears only with application of water (Picture 7,8 red curves). In this case the Method of standard Porosimetry is the only method, which can give information about porous structure.
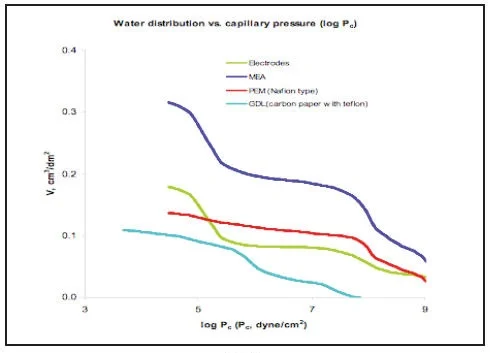
Water distribution vs. capillary pressure (Picture 9) is important for optimization of Water Management in the Fuel cell.
PICTURE 9
PICTURE 10
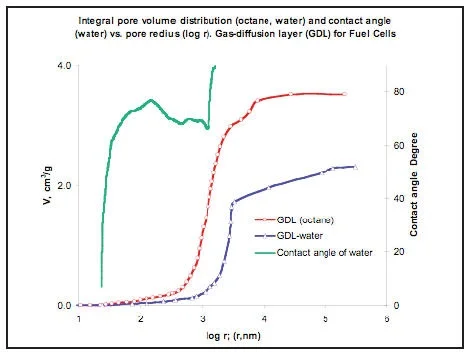
MSP can also be used for evaluation of the contact angle and the hydrophilic-hydrophobic properties of multi-component materials. For this purpose porograms with the test liquid, whose contact angle has to be determined, and with a standard liquid whose contact
angle is ~ 0° are measured. The Picture 10 shows porograms for gas - diffusion layer (GDL) in PEM fuel cell obtained with octane and water (red and blue). Here there is a shift of the curves. Contact angle is responsible for this shift. Contact angle can be easily calculated from this data (green curve). The contact angles depend on the pore radius. There is new information, which we obtained first.
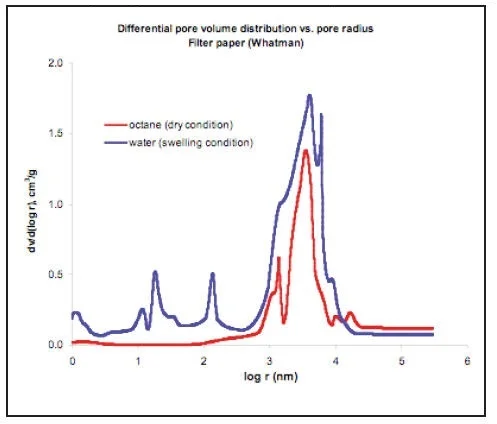
Other porous materials especially having an organic origin (leather, wood, paper, natural textile and soil) are prone to swelling in water. MSP enables examination of structural changes of swelling. Picture 11 shows differential porograms for Whatman Filter Paper. The dry structure of the Paper was studied by using octane. The structure of the Paper, as it works during filtration of water solutions, was examined by using water. It can be seen that, as a result of swelling in water, the porosity of the Paper was sharply extended.
PICTURE 11
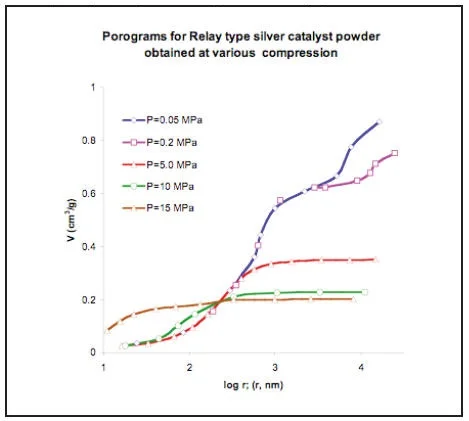
PICTURE 12
MSP gives the possibility to study the porous structure of compressible materials at various level of compression. It is possible to separate primary (inside particles) and secondary (between particles) porous structure for powders as well. An intersection of the curves showed on picture 12 characterizes border between primary and secondary structures.
To learn more about Services and Products provided by MPM&P Research Inc. please click the Button below.